Efficacy of Regorafenib in Hepatocellular Carcinoma Patients: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
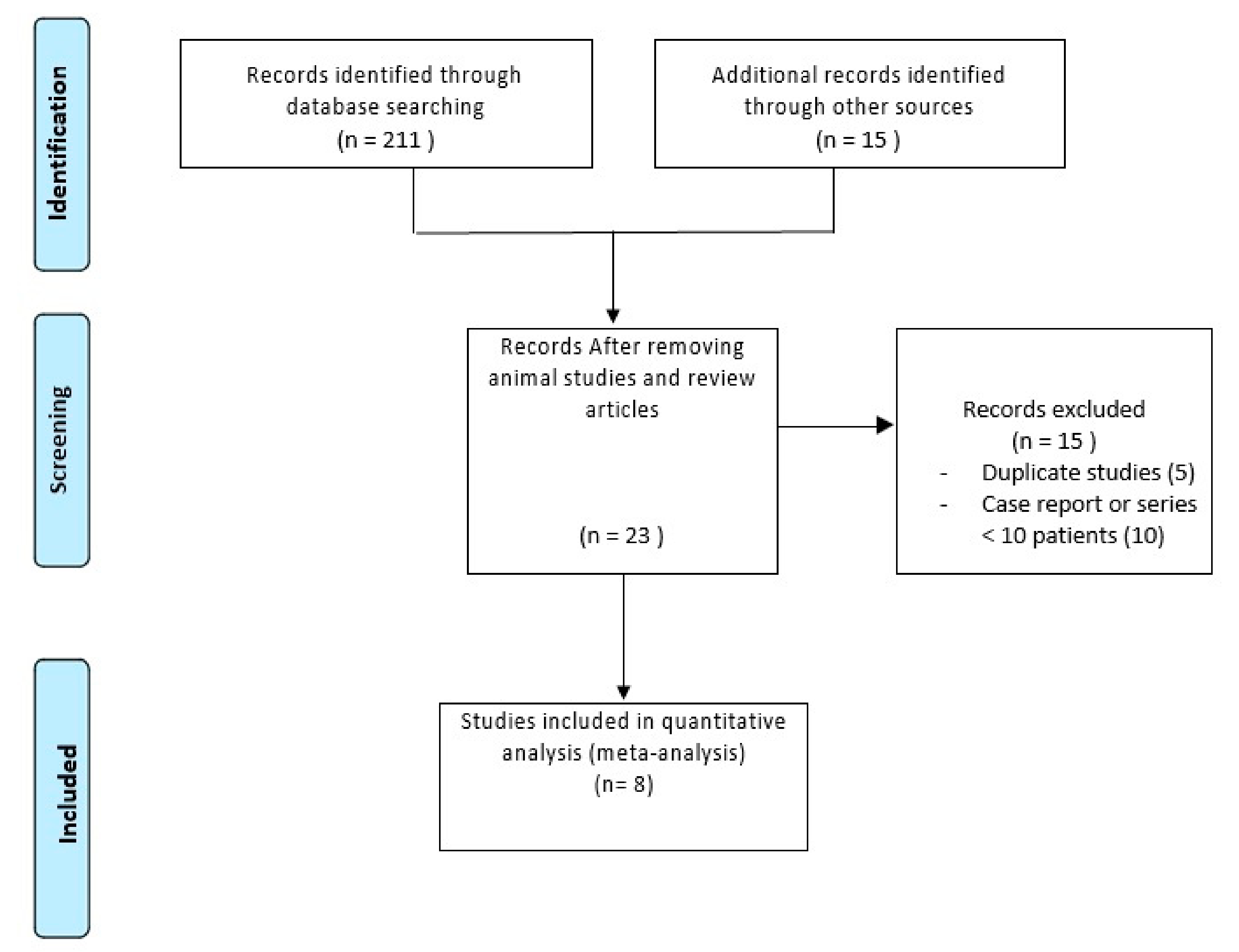
2.1. Literature Search
2.2. Characteristics of Included Studies
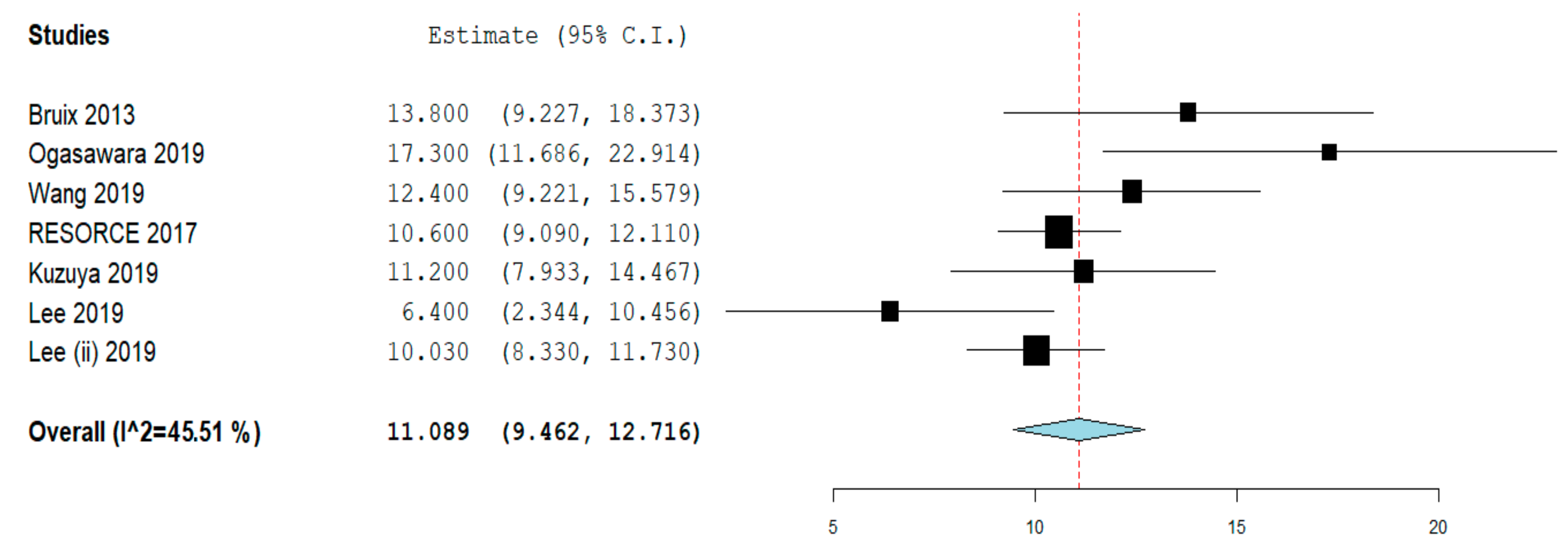
2.3. Overall Survival
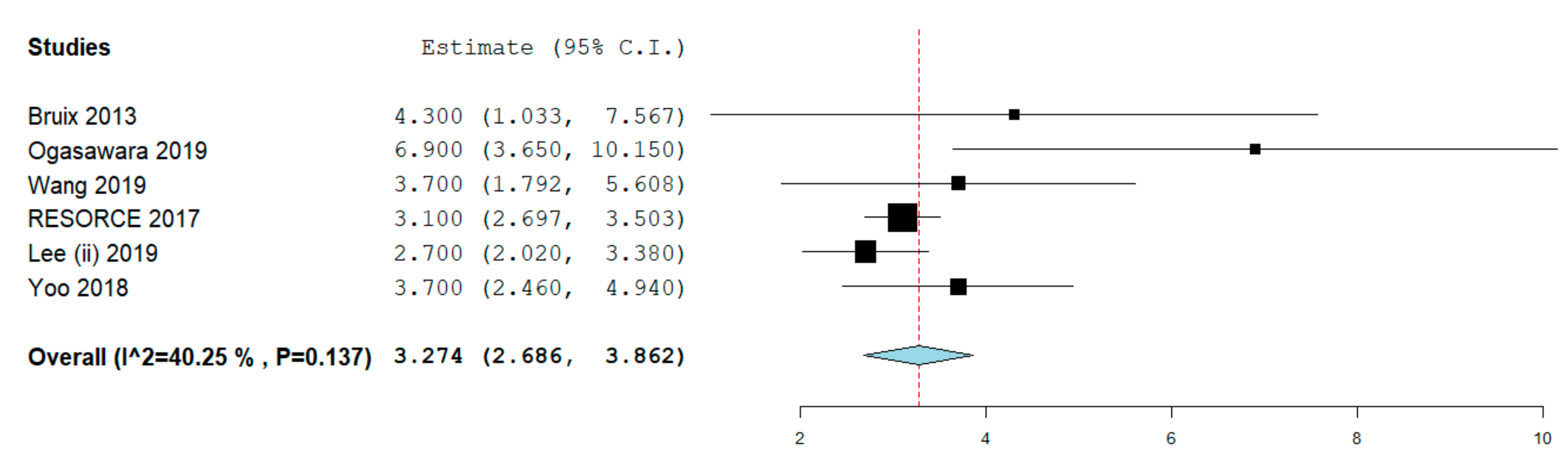
2.4. Progression-Free Survival
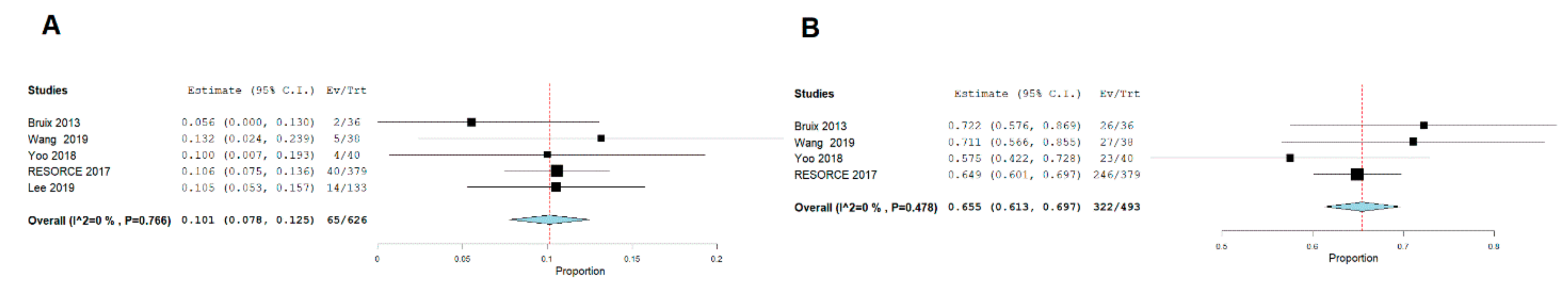
2.5. Complete Response
2.6. Major Complications
3. Discussion
4. Materials and Methods
4.1. Search Strategy and Selection Criteria
4.2. Outcomes
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- El-Serag, H.B. Hepatocellular carcinoma. New Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [Green Version]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. New Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Dumas, J.; Adnane, L.; Lynch, M.; Carter, C.A.; Schütz, G.; Thierauch, K.H.; Zopf, D. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 2011, 129, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elkacem, L.; Arns, S.; Brix, G.; Gremse, F.; Zopf, D.; Kiessling, F.; Lederle, W. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol. Cancer Ther. 2013, 12, 1322–1331. [Google Scholar] [CrossRef] [Green Version]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Demetri, G.D.; Reichardt, P.; Kang, Y.K.; Blay, J.Y.; Rutkowski, P.; Gelderblom, H.; Hohenberger, P.; Leahy, M.; Von Mehren, M.; Joensuu, H.; et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Chia, S.K.; Speers, C.H.; D’yachkova, Y.; Kang, A.; Malfair-Taylor, S.; Barnett, J.; Coldman, A.; Gelmon, K.A.; O’Reilly, S.E.; Olivotto, I.A. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer 2007, 110, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Rimola, J.; Torres, F.; Darnell, A.; Rodriguez-Lope, C.; Forner, A.; Llarch, N.; Ríos, J.; Ayuso, C.; Bruix, J. Postprogression survival of patients with advanced hepatocellular carcinoma: Rationale for second-line trial design. Hepatology 2013, 58, 2023–2031. [Google Scholar] [CrossRef]
- Facciorusso, A.; Del Prete, V.; Antonino, M.; Crucinio, N.; Neve, V.; Di Leo, A.; Carr, B.I.; Barone, M. Post-recurrence survival in hepatocellular carcinoma after percutaneous radiofrequency ablation. Dig. Liver Dis. 2014, 46, 1014–1019. [Google Scholar] [CrossRef]
- Iavarone, M.; Invernizzi, F.; Czauderna, C.; Sanduzzi-Zamparelli, M.; Bhoori, S.; Amaddeo, G.; Manini, M.A.; López, M.F.; Anders, M.; Pinter, M.; et al. Preliminary experience on safety of regorafenib after sorafenib failure in recurrent hepatocellular carcinoma after liver transplantation. Am. J. Transplant. 2019, 19, 3176–3184. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Tak, W.Y.; Gasbarrini, A.; Santoro, A.; Colombo, M.; Lim, H.Y.; Mazzaferro, V.; Wiest, R.; Reig, M.; Wagner, A.; et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicenter, open-label, phase II safety study. Eur. J. Cancer. 2013, 49, 3412–3419. [Google Scholar] [CrossRef]
- Ogasawara, S.; Ooka, Y.; Itokawa, N.; Inoue, M.; Okabe, S.; Seki, A.; Haga, Y.; Obu, M.; Atsukawa, M.; Itobayashi, E.; et al. Sequential therapy with sorafenib and regorafenib for advanced hepatocellular carcinoma: A multicenter retrospective study in Japan. Investig. New Drugs 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tsuchiya, K.; Kurosaki, M.; Yasui, Y.; Inada, K.; Kirino, S.; Yamashita, K.; Sekiguchi, S.; Hayakawa, Y.; Osawa, L.; et al. Sorafenib-Regorafenib Sequential Therapy in Japanese Patients with Unresectable Hepatocellular Carcinoma-Relative Dose Intensity and Post-Regorafenib Therapies in Real World Practice. Cancers 2019, 11, 1517. [Google Scholar] [CrossRef] [Green Version]
- Yoo, C.; Park, J.W.; Kim, Y.J.; Kim, D.Y.; Yu, S.J.; Lim, T.S.; Lee, S.J.; Ryoo, B.Y.; Lim, H.Y. Multicenter retrospective analysis of the safety and efficacy of regorafenib after progression on sorafenib in Korean patients with hepatocellular carcinoma. Investig. New Drugs 2019, 37, 567–572. [Google Scholar] [CrossRef]
- Kuzuya, T.; Ishigami, M.; Ito, T.; Ishizu, Y.; Honda, T.; Ishikawa, T.; Hirooka, Y.; Fujishiro, M. Clinical characteristics and outcomes of candidates for second-line therapy, including regorafenib and ramucirumab, for advanced hepatocellular carcinoma after sorafenib treatment. Hepatol. Res. 2019, 49, 1054–1065. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, Y.B.; Kim, M.A.; Jang, H.; Kim, S.W.; Cho, E.J.; Lee, J.H.; Yu, S.J.; Yoon, J.H.; Kim, Y.J. Regorafenib versus nivolumab for hepatocellular carcinoma patients who experienced sorafenib treatment failure: A propensity score analysis. Hepatology 2019, 70, 212A–213A. [Google Scholar]
- Lee, M.J.; Chang, S.W.; Lee, H.S.; Kim, S.; Lee, Y.S.; Jung, Y.K.; Suh, S.J.; Kim, J.H.; Seo, Y.S.; Yim, H.J.; et al. Multicenter retrospective analysis of the efficacy of regorafenib after progression on sorafenib with hepatocellular carcinoma. Hepatology 2019, 70, 239A. [Google Scholar]
- Ponziani, F.R.; Bhoori, S.; Germini, A.; Bongini, M.; Flores, M.; Sposito, C.; Facciorusso, A.; Gasbarrini, A.; Mazzaferro, V. Inducing tolerability of adverse events increases sorafenib exposure and optimizes patient’s outcome in advanced hepatocellular carcinoma. Liver Int. 2016, 36, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Torres, F.; Rodriguez-Lope, C.; Forner, A.; LLarch, N.; Rimola, J.; Darnell, A.; Ríos, J.; Ayuso, C.; Bruix, J. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J. Hepatol. 2014, 61, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Licinio, R.; Carr, B.I.; Di Leo, A.; Barone, M. MEK 1/2 inhibitors in the treatment of hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Serviddio, G.; Muscatiello, N. Transarterial radioembolization vs chemoembolization for hepatocarcinoma patients: A systematic review and meta-analysis. World J. Hepatol. 2016, 8, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Rognoni, C.; Ciani, O.; Sommariva, S.; Facciorusso, A.; Tarricone, R.; Bhoori, S.; Mazzaferro, V. Trans-arterial radioembolization in intermediate-advanced hepatocellular carcinoma: Systematic review and meta-analyses. Oncotarget 2016, 7, 72343–72355. [Google Scholar] [CrossRef]
- Rognoni, C.; Ciani, O.; Sommariva, S.; Bargellini, I.; Bhoori, S.; Cioni, R.; Facciorusso, A.; Golfieri, R.; Gramenzi, A.; Mazzaferro, V.; et al. Trans-arterial radioembolization for intermediate-advanced hepatocellular carcinoma: A budget impact analysis. BMC Cancer 2018, 18, 715. [Google Scholar] [CrossRef]
- Casadei Gardini, A.; Scarpi, E.; Faloppi, L.; Scartozzi, M.; Silvestris, N.; Santini, D.; de Stefano, G.; Marisi, G.; Negri, F.V.; Foschi, F.G.; et al. Immune inflammation indicators and implication for immune modulation strategies in advanced hepatocellular carcinoma patients receiving sorafenib. Oncotarget 2016, 7, 67142–67149. [Google Scholar]
- Casadei Gardini, A.; Marisi, G.; Scarpi, E.; Scartozzi, M.; Faloppi, L.; Silvestris, N.; Masi, G.; Vivaldi, C.; Brunetti, O.; Tamberi, S.; et al. Effects of metformin on clinical outcome in diabetic patients with advanced HCC receiving sorafenib. Expert Opin. Pharmacother. 2015, 16, 2719–2725. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; Higgins, J.P.T.; Green, S. (Eds.) The Cochrane Collaboration: Hoboken, NJ, USA, 2011; Available online: www.cochrane-handbook.org (accessed on 11 September 2019).
- Wells, G.A.; Shea, B.; O’connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle—Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 11 September 2019).
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [Green Version]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
| Study | Design; Country; Recruitment Period | Intervention | Sample Size (n) | Age (years) | Sex (male) | CP (A/B/C) | ECOG PS (0/≥1) | BCLC Stage (A/B/C) | Etiology of Liver Disease (Viral) | Baseline AFP |
|---|---|---|---|---|---|---|---|---|---|---|
| Bruix, 2013 [15] | Phase II, single arm, open label; multicenter; 2009–2012 | 160 mg orally once daily for the first 3 weeks of each 4-week cycle, followed by 1 week off treatment for: median (range) weeks: 19.5 (2–103) | 36 | 61 (40–76) | 32 (89%) | 36 (100%)/0/0 | 28 (78%)/8 (22%) | 0/4 (11%)/32 (89%) | HBV: 14 (39%), HCV: 13 (36%) | |
| Ogasawara, 2019 [16] | Retrospective study: Multicenter, Japan; before March 2018 | 160 mg regorafenib orally once per day for 3 weeks, followed by 1 week of no treatment for each cycle. 30 patients (68.2%) had initial dose of regorafenib of 160 mg; for 5.7 months (95% CI: 1.82–9.5) | 44 | 71 (60–85) | 38 (86.4%) | 40 (91%)/4(9%)/0 | ≤1: 44 (100.0) | C: 34 (77.3) | HBV: 7 (15.9%), HCV: 15 (34.1%) | >400 ng/mL: 17 (38.6) |
| Wang 2019 [17] | Retrospective study; single center, Japan; July 2017 to June 2019 | 160 mg orally once per day for 3 weeks, followed by 1 week of no treatment for each cycle; for median duration of 2.6 months | 38 | 75 (31–88) | 32 (84%) | 33 (87%)/5 (13%)/0 | 17 (45%)/21 (55%) | 0/17(45%)/21 (55%) | HBV: 7 (18%), HCV: 16 (43%) | Median (range), ng/mL: 174.2 (2.6–448620); Baseline AFP >400: 16 (42%) |
| Yoo 2018 [18] | Retrospective study; multicentre; Korea; April 2017 to August 2017 | 40 | 62 (39–83) | 36 (90%) | 36 (90%)/3 (8%)/1 (2%) | 7 (18%)/33 (82%) | 0/6 (15%)/34 (85%) | HBV: 27 (67%), HCV: 2 (5%) | ≥400 ng/mL: 16 (40%) | |
| RESORCE trial, 2017 [9] | Randomized, double-blind, placebo-controlled, phase III trial; multinational (21 countries; 152 centers) study; May 14, 2013 to December 31, 2015 | Regorafenib: 379; Placebo: 194 | Median (IQR) years: Regorafenib group: 64 (54–71), Placebo group: 62 (55–68) | Regorafenib group: 333 (88%), Placebo group: 171 (88%) | Regorafenib: 373 (98%)/5 (1%)/0; Placebo group: 188 (97%)/6 (3%)/0 | Regorafenib: 247 (65%)/132 (35%). Placebo: 130 (67%)/64(33%)/0 | Regorafenib: 1 (< 1%)/53 (14%)/325 (86%). Placebo: 0/22 (11%)/172 (89%) | Regorafenib group: *HBV: 143 (38%), *HCV: 78 (21%); Placebo group: *HBV: 73 (38%), *HCV: 41 (21%) | ≥400 ng/mL: Regorafenib: 162 (43%), Placebo: 87 (45%). | |
| Kuzuya, 2019 [19] | Retrospective study; single center; Japan; between June 2011 and December 2016 | 36 | Age <69 years: 19 patients (52.8%) | 32 (88.9%) | A: 27 (75%)/ B &C: 9 (25%) | 28 (77.8%)/8(22.2%) | B: 9 (25%) | <400 ng/mL: 27 (75%) | ||
| Lee, 2019 [20] | Retrospective study (propensity score matching); single center; Korea, 2015–2018 | 103 | ||||||||
| Lee, 2019 (ii) [21] | Retrospective study; Multicenter; Korea; 2017–2019 | 133 | 60 years | 112 (84.2%) | 111/ 1/ 1 | HBV: 91 (68.4%) |
| Variable | Subgroup | No. of Cohorts | No. of Patients | Summary Estimate (95% CI) | Within-Group Heterogeneity (I2) |
|---|---|---|---|---|---|
| Overall Survival | |||||
| Study design | Randomized trial | 1 | 379 | 13.8 (9.2–18.4) | NA |
| Retrospective | 6 | 390 | 11.4 (9.1–13.6) | 61% | |
| Study quality | Low/moderate | 4 | 310 | 10.2 (8.2–12.2) | 46.5% |
| High | 3 | 459 | 13.2 (9.2–17.1) | 68.5% | |
| Patients recruitment | Single center | 3 | 177 | 10.2 (6.9–13.5) | 63.5% |
| Multicenter | 4 | 592 | 11.6 (9.5–13.7) | 60.8% | |
| Progression-free Survival | |||||
| Study design | Randomized trial | 1 | 379 | 4.3 (1.03–7.56) | NA |
| Retrospective | 5 | 291 | 3.65 (2.6–4.7) | 52% | |
| Study quality | Low/moderate | 3 | 274 | 3.4 (1.9–4.9) | 48.3% |
| High | 4 | 499 | 3.74 (2.6–4.8) | 52% | |
| Patients recruitment | Single center | 2 | 158 | 4.4 (2.08–6.8) | 28.2% |
| Multicenter | 5 | 632 | 3.27 (2.6–3.9) | 50% | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facciorusso, A.; Abd El Aziz, M.A.; Sacco, R. Efficacy of Regorafenib in Hepatocellular Carcinoma Patients: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 36. https://doi.org/10.3390/cancers12010036
Facciorusso A, Abd El Aziz MA, Sacco R. Efficacy of Regorafenib in Hepatocellular Carcinoma Patients: A Systematic Review and Meta-Analysis. Cancers. 2020; 12(1):36. https://doi.org/10.3390/cancers12010036
Chicago/Turabian StyleFacciorusso, Antonio, Mohamed A. Abd El Aziz, and Rodolfo Sacco. 2020. "Efficacy of Regorafenib in Hepatocellular Carcinoma Patients: A Systematic Review and Meta-Analysis" Cancers 12, no. 1: 36. https://doi.org/10.3390/cancers12010036






